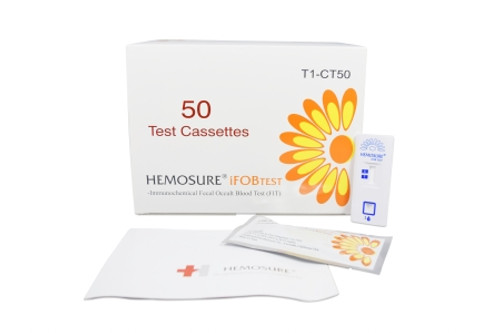
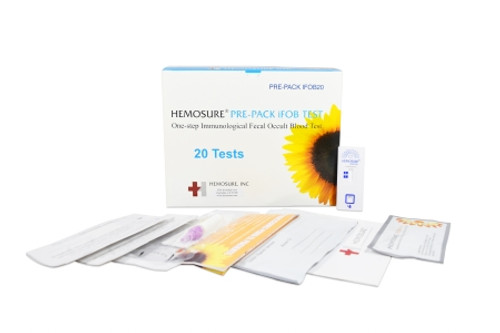
Product Overview
Manufacturer
- QUIDELORTHO SALE
Features
- The QuickVue iFOB (immunochemical Fecal Occult Blood) Test is an immunochemical device intended for the qualitative detection of fecal occult blood by laboratories or physicians* offices
- The QuickVue iFOB Test is a one-step lateral flow chromatographic immunoassay
- Bold burgundy test results are easy to read and interpret
Attributes
- Application: Cancer Screening Test Kit
- CLIA Classification: CLIA Waived
- Contents 1: (20) Individually Wrapped Test Cassettes, Package Insert, Procedure Card, Specimen Collection Tube with 2 mL FOB Buffer, Specimen Collection Paper with Adhesive, Specimen Pouch, Absorbent Sleeve, Return Mailer, Patient Instructions
- Is_Active_Vendor: Y
- Is_DSCSA: N
- Is_Discontinued: N
- Is_ECAT: N
- Is_GSA: Y
- Is_Medical_Device: N
- Is_VA: N
- Lot_Tracking_Flag: N
- Number of Tests: 20 Tests
- On_Allocation: N
- Product Dating: McKesson Acceptable Dating: we will ship >= 180 days
- Purchase Program Type: Standard Purchase
- Reading Type: Visual Read
- Sample Type: Stool Sample
- Specialty: Immunoassay
- Supplier_ID: 487993
- Test Format: Cassette Format
- Test Kit Type: Rapid
- Test Method: Lateral Flow Method
- Test Name: Fecal Occult Blood Test (iFOB or FIT)
- Test Type: Colorectal Cancer Screening
- Time to Results: 5 to 10 Minute Results
- UNSPSC Code: 41116120
- Latex Free Indicator: Not Made with Natural Rubber Latex