Product Overview
Manufacturer
- QUIDELORTHO SALE
Features
- Sofia? SARS Antigen FIA test is only for use under the Food and Drug Administration*s Emergency Use Authorization: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
- Product ships with minimum 30 days dating
- Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. ?263a, that meet the requirements to perform moderate, high or waived complexity tests
- This test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
- The SARS Antigen FIA does not differentiate between SARS-CoV and SARS-CoV-2
- Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status
- Positive results do not rule out bacterial infection or co-infection with other viruses; the agent detected may not be the definite cause of disease
- Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities
- Negative results do not rule out COVID-19 and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions
- Negative results should be considered in the context of a patient*s recent exposures, history and the presence of clinical signs and symptoms consistent with COVID-19
- Rapid results in 15 minutes to support efficient dispositioning of patients
- Objective, accurate results without cross-reactivity to seasonal coronaviruses
- The Sofia SARS Antigen FIA is a lateral flow immunofluorescent sandwich assay that is used with the Sofia and Sofia 2 instrument
- Seamless test workflow follows a similar format to CLIA-waived Sofia and Sofia 2 assays
- Dual work modes adjust to volume fluctuations and allows for significant throughput and batching of samples in READ NOW Mode
- Fluorescent technology with automated read eliminates the subjectivity of a visual result
- Virena provides automated tracking, data capture, government reporting, and exclusive disease mapping
- All necessary components included in kit, ready for use for nasal swab procedure
- Self-contained Test Cassette that is clean, easy to use and dispose of
- For in vitro diagnostic use; RX only
Attributes
- Application: Respiratory Test Kit
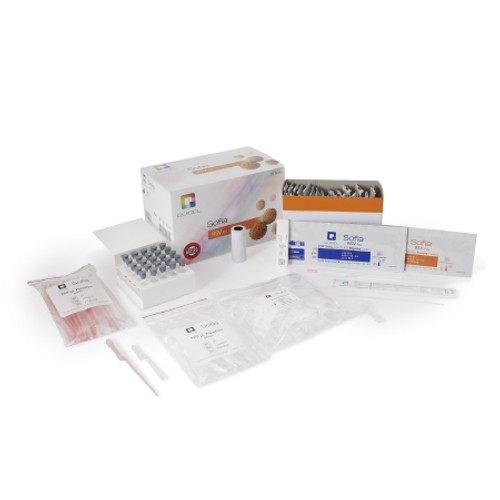
- Contents 1: (25) Individually Packaged Test Cassettes, (25) Reagent Tubes, (25) Ampoules of Reagent Solution, (25) Sterile Nasal Swabs, (25) X 120 ?L Fixed Volume Pipettes, Positive Control Swab, Negative Control Swab, Package Insert, Quick Reference Instructions, QC Card
- For Use With: For use with Sofia / Sofia 2 Instrument
- Is_Active_Vendor: Y
- Is_DSCSA: N
- Is_Discontinued: N
- Is_ECAT: N
- Is_GSA: Y
- Is_Medical_Device: N
- Is_VA: N
- Lot_Tracking_Flag: Y
- Number of Tests: 25 Tests
- On_Allocation: N
- Product Dating: McKesson Acceptable Dating: we will ship >= 30 days
- Purchase Program Type: Standard Purchase
- Reading Type: Machine Read
- Sample Type: Nasal Swab / Nasopharyngeal Swab Sample
- Specialty: Immunoassay
- Supplier_ID: 487993
- Technology: Immunofluorescent Sandwich Assay
- Test Format: Cassette Format
- Test Kit Type: Rapid
- Test Name: SARS Antigen FIA
- Test Type: Fluorescence Immunoassay (FIA)
- Time to Results: 15 Minute Results
- UNSPSC Code: 41116205