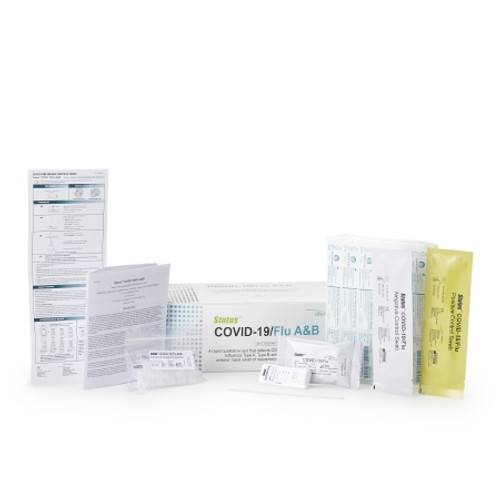
Product Overview
Manufacturer
- LIFESIGN LLC
Features
- Status* COVID-19 / FLU A and B test has been granted emergency use authorization (EUA) by the FDA for use at the point-of-care setting: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
- Product ships from McKesson with minimum 30 days dating
- Status* COVID-19/Flu test is a lateral flow immunoassay intended for the in vitro rapid, simultaneous qualitative detection and differentiation of nucleocapsid antigen from SARS-CoV-2, influenza A and influenza B directly from nasopharyngeal swab specimens obtained from individuals, who are suspected of respiratory viral infection consistent with COVID-19 by their healthcare provider, within the first five days of onset of symptoms
- Results are for the simultaneous identification of nucleocapsid antigens of SARS-CoV-2, influenza A and influenza B, but does not differentiate between SARS-CoV and SARS-CoV-2 viruses and is not intended to detect influenza C antigens
- Positive results indicate the presence of viral antigens, but the clinical correlation with patient history and other diagnostic information is necessary to determine infection status
- Positive results do not rule out bacterial infection or co-infection with other viruses
- Laboratories within the United States and its territories are required to report all SARS-CoV-2 results to the appropriate public health authorities
- Negative SARS-CoV-2 results should be treated as presumptive and confirmed with a molecular assay, if necessary, for patient management
- Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions
- Negative results should be considered in the context of a patient*s recent exposures, history, and the presence of clinical signs and symptoms consistent with COVID-19
- The Status* COVID-19/Flu test is intended for use by medical professionals and laboratory personnel trained to perform the test
- Flocked nasopharyngeal swab for superior specimen collection and patient comfort
- COVID-19 - Sensitivity 93.9%, Specificity 100%
- Flu A - Sensitivity 91.4%, Specificity 95.7%
- Flu B - Sensitivity 87.6%, Specificity 95.9%
- During the duration of the emergency this test can be performed in a patient care setting that is operating under a CUA Waiver, Certificate of Compliance or Certificate of Accreditation
Attributes
- Application: Respiratory Test Kit
- Contents 1: (25) Test Devices, (25) Extraction Reagent Capsules, (25) Sterile Swabs, Positive Control Swab, Negative Control Swab, Package Insert, Quick Reference Instruction
- Is_Active_Vendor: Y
- Is_DSCSA: N
- Is_Discontinued: N
- Is_Medical_Device: N
- Lot_Tracking_Flag: Y
- Number of Tests: 25 Tests
- On_Allocation: N
- Product Dating: McKesson Acceptable Dating: we will ship >= 30 days
- Purchase Program Type: Standard Purchase
- Reading Type: Visual Read
- Sample Type: Nasopharyngeal Swab Sample
- Specialty: Immunoassay
- Supplier_ID: 487886
- Test Format: Test Device Format
- Test Kit Type: Rapid
- Test Method: Lateral Flow Immunoassay
- Test Name: COVID-19 / Flu A and B
- Test Type: Antigen Detection
- Time to Results: 15 Minute Results
- UNSPSC Code: 41116144